Polybenzofulvene derivatives bearing dynamic binding sites as potential anticancer drug delivery systems†
Abstract
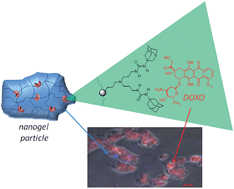
In order to obtain new advanced functional materials capable of recognizing drug molecules, the polybenzofulvene backbone of molecular brush poly-6-MOEG-9-TM-BF3k has been functionalized with a “synthetic dynamic receptor” composed of two 1-adamantylurea moieties linked together by means of a dipropyleneamino bridge as in Meijer's bis(adamantylurea) pincer (BAUP). This functional material, bearing synthetic receptors potentially capable of recognizing/loading and then delivering drug molecules, was used to prepare colloidal drug delivery systems (by means of soft interaction with BAUP) for delivering the model anti-cancer drug doxorubicin (DOXO). The resulting nanostructured drug delivery systems containing the physically loaded drug were characterized in terms of drug loading and release, dimensions and zeta potential, and in vitro cell activity and uptake on two different cell lines (i.e. the human bronchial epithelial 16HBE and the human colon cancer HCT116). On normal cells, free DOXO was found to be more cytotoxic than DOXO-loaded nanogels at the higher tested concentration and, only on cancer cells, DOXO-loaded nanogels show similar or slightly higher cytotoxicity values than free DOXO, suggesting potential advantages in the treatment of cancer. These results were supported by fluorescence microscopy studies, which suggested that DOXO-loaded nanogels provide an extracellular reservoir of the drug, which is gradually released and internalized within the cells.

 Please wait while we load your content...
Please wait while we load your content...