In vitro and in vivo assessments of a 3-(3,4-dihydroxyphenyl)-2-propenoic acid bioconjugated gelatin-based injectable hydrogel for biomedical applications†
Abstract
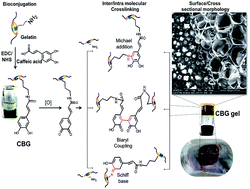
Imparting functional properties on a biomaterial for high end applications is always a challenging task. In the present study, an attempt was made to construct an injectable hydrogel through bioconjugation of dihydroxy phenolic acids to a gelatin backbone. Bioconjugating caffeic acid with gelatin followed by oxidation with mild oxidation agents provided a hydrogel with all the requisite properties (biocompatibility, controlled biodegradability, and antioxidant, antimicrobial and wound healing ability). Bioconjugation was performed using EDC/NHS and the resultant gel named as caffeic acid bioconjugated gel (CBG gel). The physicochemical, rheological, swelling, in vitro (biocompatibility, biodegradability, antimicrobial properties, antioxidant properties and drug release properties) and in vivo (biocompatibility, biodegradability and wound healing properties) studies on the CBG gel were carried out using standard protocols. The bioconjugation was confirmed by 1H NMR and UV-Vis analysis. Rheological analysis of the CBG gel revealed that the storage modulus was greater than the loss modulus at all the frequencies and suggested the elastic nature of the gel. About 50% weight gain within 12 hours during swelling studies and 50% weight loss within 12 hours during evaporation suggested the suitability of the CBG gel as a drug carrier. The drug release studies implied that there was an initial burst and later the release was sustained. The CBG gel promotes cell migration and demonstrates radical scavenging behavior. When subcutaneously injected into the animal, as in situ CBG gel, the gel was highly biocompatible and did not cause any necrosis. The crosstalk with adjacent tissue cells was smooth and the gel completely degraded within 24 days. The wound healing efficacy on full-thickness wounds suggested that the CBG gel accelerated healing and imparted high strength on the healed skin at an appreciable level. With all these additional functional properties, the CBG gel could be useful for biomedical applications.

 Please wait while we load your content...
Please wait while we load your content...