Synthesis and characterization of a biocompatible monotyrosine-based polymer and its interaction with DNA†
Abstract
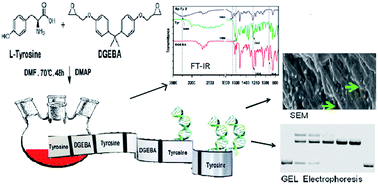
A novel tyrosine-based copolymer containing L-tyrosine (Tyr) and diglycidylether of bisphenol A(DGEBA) was synthesized and studied for its interaction with DNA for potential applications in biological systems. The synthesis of the polymer was optimized by varying monomer ratios using 4-(dimethylamino)pyridine (DMAP) as a catalyst to yield polymers with a Mw of 7500–8000. Further characterization by FTIR, NMR and thermal analysis supported the formation of the monotyrosine–DGEBA polymer. The interaction of the 1 : 1 DGEBA–tyrosine copolymer with DNA was investigated by gel electrophoresis, thermal melting, and fluorescence spectroscopy in ratios ranging from 0.5 : 1 to 12 : 1 polymer–DNA (w/w). The copolymer was seen to lend stability to the DNA without damaging it and demonstrated endonuclease resistivity that is conducive for biological applications. Scanning electron microscopy, dynamic light scattering and zeta potential studies of the polymer–DNA complex also established that the polymer is capable of encapsulating DNA leading to the formation of the DNA–polymer polyplex nano-assembly. The potential of the polymer for biological applications was further reinstated by its non-cytotoxicity.

 Please wait while we load your content...
Please wait while we load your content...