Water dispersible Ag@polyaniline-pectin as supercapacitor electrode for physiological environment†
Abstract
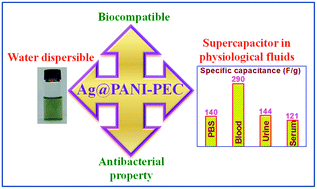
Designing the supercapacitor electrode material for implantable electronic medical devices (IEMDs) requires careful consideration because of the need for materials which are inherently high in capacitance, biocompatibility, and antibacterial activity and are able to work in physiological environment. For the first time, we report the synthesis of a nanocomposite which has the aforementioned properties and demonstrate the nanocomposite as a supercapacitor electrode material operating in physiological fluids. In the first step, water dispersible polyaniline-pectin (PANI-PEC) nanoparticles were synthesized using biopolymer pectin (PEC) as the stabilizer. In the second step, the synthesized PANI-PEC was treated with a silver nitrate solution to afford silver nanoparticles (Ag NPs) decorated PANI-PEC nanocomposite (Ag@PANI-PEC). PANI-PEC acted as a reducing agent to convert silver ions to Ag NPs, thus eliminating the need of an exogenous reducing agent. Ag@PANI-PEC displays a specific capacitance of 140, 290, 144 and 121 F g−1 in phosphate buffer saline, blood, urine and serum, respectively, which are all physiological fluids. Furthermore, due to the use of biopolymer PEC, PANI-PEC and Ag@PANI-PEC exhibited biocompatibility and the presence of silver on Ag@PANI-PEC rendered antibacterial properties to the latter, thus making them an ideal material for in vivo implants. These findings establish the feasibility of using the nanocomposite as a potential material for energy storage device in IEMDs.

 Please wait while we load your content...
Please wait while we load your content...