A novel chitosan–collagen-based hydrogel for use as a dermal filler: initial in vitro and in vivo investigations
Abstract
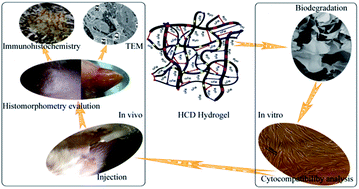
Novel hydrogels (termed HCD hydrogels) were synthesized based on human-like collagen (HLC) and chitosan (CS) cross-linked with dialdehyde starch (DAS). The biological stability and biocompatibility of HCD hydrogels were determined through in vitro and in vivo tests. The mechanism of hydrogel formation was studied using Fourier transform infrared spectroscopy (FTIR), which showed that covalent bonds formed via acetalization and Schiff base reactions. Biological stability was evaluated in vitro by degrading HCD hydrogels with class I collagenase, class II collagenase, and both class I and class II collagenases and in vivo after subcutaneously injecting HCD into an animal model. The biological characteristics of HCD hydrogels was studied by two methods: (i) MTT and cytomorphology cytotoxicity and cytocompatibility and (ii) in vivo, whereby histomorphometry, transmission electron microscopy (TEM), and immunohistochemistry were used to compare different types of surgically introduced hydrogels, our HCD hydrogels, SunMax Collagen Implant hydrogels (SUM hydrogels), and OUTLINE&EVOLUTION Injectable Synthetic Gel hydrogels (EVL hydrogels). The in vivo analyses were performed at 1, 9, 12, and 28 weeks after surgery. The hydrogel biodegradation results showed that the normalized residual weight (WR) of HCD hydrogels varied with DAS content. In vitro, we found that the minimum WR of HCD hydrogels was 42.19% after 28 weeks when degraded by both types of class I and class II collagenase. The MTT assay indicated that the minimum relative growth rate (RGR) of cells was 93% after they were incubated with HCD hydrogels for 7 days, suggesting good cytocompatibility. In vivo histomorphometry results indicated that HCD hydrogels effectively filled tissue voids and did not cause redness, edema, festering, or color changes. In addition, a few vessels grew into the hydrogel and a thin fibrous capsule was eventually produced. TEM and immunohistochemistry studies suggested that HCD hydrogels produced less intense inflammatory responses than those produced by SUM hydrogels and EVL hydrogels. Overall, HCD hydrogels afford both enhanced biological stability and excellent biocompatibility, making them potentially promising for skin patch scaffolds, wrinkle treatments, and tissue cavity fillers.

 Please wait while we load your content...
Please wait while we load your content...