Multiscale evaluation of pore curvature effects on protein structure in nanopores†
Abstract
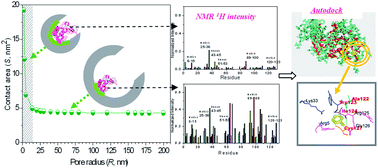
Protein structure in nanopores is an important determinant in porous substrate utilization in biotechnology and materials science. To date, accurate residue details of pore curvature induced protein binding and unfolding were still unknown. Here, a multiscale ensemble of chromatography, NMR hydrogen and deuterium (H/D) exchange, confocal scanning and molecular docking simulations was combined to obtain the protein adsorption information induced by pore size and curvature. Lysozyme and polystyrene microspheres within pores in the 14–120 nm range were utilized as models. With pore size increasing, the bound lysozyme presented a tendency of significantly decreased retention, less unfolding and fewer interacted sites. However, such a significant dependence between pore curvature and protein size only existed in a limited micro-pore range comparable to protein sizes. The mechanism behind the above events could be attributed to the diverse protein interaction area determined by pore curvature and size change, by models calculating the binding of lysozyme onto surfaces. Another surface of opposite curvature for nanoparticles was also calculated and compared, the rules were similar but with opposite direction and such a critical size also existed. These studies of proteins on curved interfaces may ultimately help to guide the design of novel porous materials and assist in the discrimination of the target protein from molecular banks.

 Please wait while we load your content...
Please wait while we load your content...