Injectable and biodegradable supramolecular hydrogels formed by nucleobase-terminated poly(ethylene oxide)s and α-cyclodextrin†
Abstract
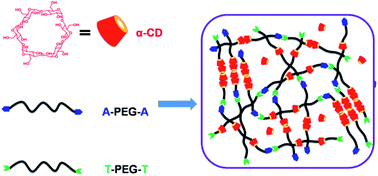
Injectable and biodegradable supramolecular hydrogels were prepared by nucleobase (adenine/thymine)-terminated poly(ethylene oxide)s (A-PEG-A/T-PEG-T) and α-cyclodextrin (α-CD). The supramolecular hydrogels were thoroughly characterized by WXRD, rheometer, and SEM. The gelation time depended on the molecular weight of PEG and the concentration of polymer precursors. The rheological studies showed enhanced elastic modulus (G′) of hydrogels, because of the hydrogen-bonding between A and T acting as additional network junctions. In vitro evaluation showed that the supramolecular hydrogels have acceptable biocompatibility, and are suitable for sustained and controlled release of loaded antitumor drugs. Gel formation was also confirmed when the supramolecular hydrogels were subcutaneously injected into rats. In addition, in vivo experiments employing U14 cancer cell xenograft-bearing mice showed that the intratumoral injection of a DOX-loaded A-PEG-A/T-PEG-T/α-CD gel inhibited tumor growth more effectively than that of free DOX, DOX-loaded PEG/α-CD gel, saline or gel alone. Hence, such a simple and convenient anti-cancer drug delivery system of a A-PEG-A/T-PEG-T/α-CD supramolecular hydrogel would be a promising candidate for many biomedical applications, especially in the area of the chemotherapy of solid tumors.

 Please wait while we load your content...
Please wait while we load your content...