Towards smart polymeric drug carriers: self-assembling γ-substituted polycaprolactones with highly tunable thermoresponsive behavior†
Abstract
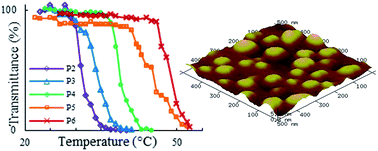
Synthesis and ring opening polymerization of a new γ-substituted ε-caprolactone monomer, γ-(2-methoxyethoxy)-ε-caprolactone is reported. Amphiphilic diblock copolymers comprised of poly[γ-(2-methoxyethoxy)-ε-caprolactone] and thermosensitive poly{γ-2-[2-(2-methoxyethoxy)ethoxy]ethoxy-ε-caprolactone} as the hydrophobic and hydrophilic blocks, respectively, were prepared. The copolymers exhibited fully biodegradable backbones and highly tunable thermoresponsive behavior in the range of 31–43 °C. Additionally, the copolymers were shown to self-assemble in aqueous media above their respective critical micelle concentrations, on the order of 10−2 g L−1. Due to their thermosensitive, self-assembling, and biodegradable properties, these copolymers demonstrate potential for the use in polymeric micellar drug delivery systems.

 Please wait while we load your content...
Please wait while we load your content...