Accurate engineering of hexagonal hollow carbon nitride with carbon vacancies: enhanced photocatalytic H2 evolution and its mechanism†
Abstract
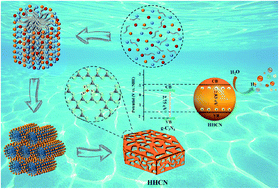
Vacancy engineering and morphology construction were integrated simultaneously by the self-assembly of melamine (MA) and cyanuric acid (CA) in the presence of P123 and H2SO4, which contributed to the hexagonal hollow carbon nitride with carbon vacancies (HHCN). P123 functioned as the structure directing agent and H2SO4 acted as the hole-maker and stability-maintainer, the synergistic effect of whom contributed to the hexagonal hollow structure. Meanwhile, the chemical oxidation etching of H2SO4 brought about carbon vacancies. Combined with experiments and DFT calculations, it was found that carbon vacancy improved the separation rate of photo-induced carriers as well as the adsorption capacity of reaction substrate (H2O molecules) and negatively shifted the CB potential. Therefore, HHCN was endowed with the superior photocatalytic hydrogen evolution rate (5140 μmol h−1 g−1), which was 18.3-fold higher than g-C3N4. Furthermore, the possible formation mechanism and photocatalytic mechanism were proposed. This work offered an excellent basis for vacancy engineering and morphology construction for the modification of carbon nitride.


 Please wait while we load your content...
Please wait while we load your content...