Electrocatalytic fixation of N2 into NO3−: electron transfer between oxygen vacancies and loaded Au in Nb2O5−x nanobelts to promote ambient nitrogen oxidation†
Abstract
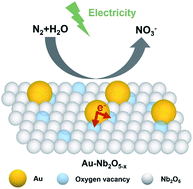
The electrocatalytic nitrogen oxidation reaction (NOR) is a promising alternative to the industrial synthesis of nitrate. However, with the enhancement of NOR activity by modification methods, the competitive oxygen evolution reaction (OER) is always improved simultaneously. Here, a hybrid electrocatalyst composed of Au nanoparticles and oxygen vacancy-enriched niobium oxide nanobelts (Au–Nb2O5−x) was prepared on niobium foil for N2 fixation into NO3−. The results of X-ray photoelectron spectroscopy (XPS) and electron paramagnetic resonance (EPR) demonstrate that electron transfer occurs between Au nanoparticles and the oxygen vacancies in Nb2O5−x nanobelts, which adjusts the electronic structures of oxygen vacancies near the Au/Nb2O5−x interface. Electrochemical and N2 temperature-programmed desorption (TPD) tests indicate that the optimization of the electronic structure suppresses the activity of the competitive OER on oxygen vacancy sites and facilitates nitrogen adsorption on the surface of Au–Nb2O5−x, which significantly enhances the NOR performance of the electrocatalyst.


 Please wait while we load your content...
Please wait while we load your content...