Facile phase transition engineering of MoS2 for electrochemical hydrogen evolution†
Abstract
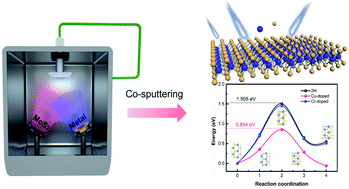
Metallic phase molybdenum disulfides exhibit impressive charge transfer ability and this has made them interesting candidates for use in the field of nano-science and heterogeneous catalysis. However, the synthesis of 100% pure bare 1T-MoS2 is difficult due to its kinetic instability. In the past, the phase transition mechanism from a semiconductor (2H) to a metallic (1T) phase has been explained only by theoretical calculations. Herein, we used magnetron sputtering to deposit a series of MoS2 films containing various metal heteroatoms as the dopants. Density functional theory (DFT) calculations revealed that the single-doped Cu-MoS2, Au-MoS2, Ag-MoS2, and Al-MoS2 exhibited distinct phase transitions compared to Cr-MoS2, Hf-MoS2, Ta-MoS2, and Zr-MoS2, due to the introduction of additional charge. Furthermore, the hydrogen evolution reaction (HER) activities of the series of MoS2 films were explored, and the adsorption free energy of H atoms was evaluated by DFT calculations.
- This article is part of the themed collection: Journal of Materials Chemistry A HOT Papers


 Please wait while we load your content...
Please wait while we load your content...