Oxygen vacancies for promoting the electrochemical nitrogen reduction reaction
Abstract
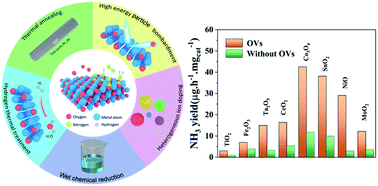
Ammonia (NH3) is a crucial chemical as a fertilizer and energy carrier, which is mainly produced by the traditional Haber–Bosch process that consumes hydrogen and leads to the emission of carbon dioxide. The electrochemical nitrogen reduction reaction (ENRR) is considered as an alternative method with low energy consumption and mild condition. However, the NH3 yield and faradaic efficiency (FE) of the electrocatalyst are dominated by the competition between the hydrogen evolution reaction (HER) and NRR. In order to achieve both high catalytic activity and high selectivity, the poor-performance catalyst needs to be improved via modification engineering. For the catalysts of oxygenated compounds, introducing oxygen vacancies (OVs) into the structure can enhance the catalytic property of NRR via regulating the local electronegativity and coordination environment. In this review, recent advances in the detection, preparation and application of OVs for NRR are summarized. We focus on the generating strategies of OVs and their evaluation method, including vacuum annealing, high-temperature hydrogen reduction, wet chemical, plasma treatment, and ion-doping strategy. Finally, the opportunities and challenges towards the suitable design of OVs in oxides for NRR are highlighted.
- This article is part of the themed collection: Journal of Materials Chemistry A Recent Review Articles


 Please wait while we load your content...
Please wait while we load your content...