Effects of surface chemical potentials on cation segregation†
Abstract
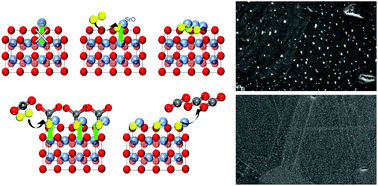
Surface cation segregation on perovskite-type electrodes is one of the major issues limiting the durability of high-temperature solid-oxide electrochemical cells, and this process is strongly dependent on temperature, the external gas-environment, and impurities in air, such as CO2. Here cation segregation on La0.6Sr0.4Co0.2Fe0.8O3−δ (LSCF) is systematically evaluated under a set of chemical potentials to determine the origin of surface segregation and the dominant factors that govern the segregation process. Temperature is the main driving force for surface strontium segregation as the thermodynamic stability of each phase varies, and the SrO particles appear primarily in a specific operating window. In addition to the required thermal energy, the presence of gaseous oxygen-containing molecules as reactants help drive the precipitation of SrO. Oxygen partial pressure (pO2) controls the defect chemistry of LSCF, leading to promotion or suppression of surface segregation. The presence of CO2 promotes the nucleation process and suppresses the surface migration step, significantly altering the surface morphology. We also show that A-site deficiency can limit the SrO segregation in certain conditions but shows no effect in others. This study reveals the impact of gas–solid interactions on surface segregation and highlights the subtle relationship between multiple segregation driving forces.


 Please wait while we load your content...
Please wait while we load your content...
