Pulsed electrodeposition of well-ordered nanoporous Cu-doped Ni arrays promotes high-efficiency overall hydrazine splitting†
Abstract
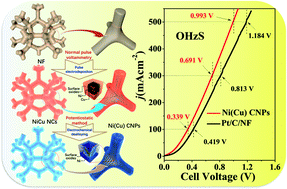
Herein, a double-hierarchical copper-doped nickel cubic nanopore (Ni(Cu) CNP) catalyst is fabricated by a normal-pulse-voltammetry electrodeposition and a subsequent in situ electrochemical etching process. As a bifunctional catalyst, the Ni(Cu) CNPs display Pt-like hydrogen evolution reaction (HER) activity and superior hydrazine oxidation reaction (HzOR) performance to deliver a current density of 10 mA cm−2 at an ultralow overpotential of 41 mV and a working potential of −18 mV, respectively. Notably, the self-assembled electrolytic hydrazine electrolyzer using the paired Ni(Cu) CNPs can achieve 10 and 504 mA cm−2 at extremely low cell voltages of 70 mV and 1.0 V, respectively, which are markedly superior to those of a Pt/C couple. The 25-fold increased electrochemically active surface area (ECSA) and the strong synergistic effect induced by Cu-doping and the construction of a NiO/Ni heterojunction all contribute to the excellent HER activity on Ni(Cu) CNPs. Additionally, the HzOR mechanism study demonstrates that hydrazine on Ni(Cu) CNPs is first transformed into diazene via a two-electron transfer pathway, and then completely oxidized to nitrogen gas by another two-electron transfer.


 Please wait while we load your content...
Please wait while we load your content...