Facile synthesis of a CuMnOx catalyst based on a mechanochemical redox process for efficient and stable CO oxidation†
Abstract
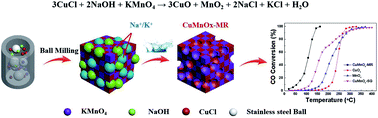
Considering the large amount of carbon monoxide produced by human activities causing serious problems to the environment, it can be seen that the low-temperature catalytic combustion of CO is a desirable route for CO removal. Herein, we reported an efficient and stable copper–manganese catalyst (CuMnOx-MR) based on a mechanochemical redox process. Interestingly, more oxygen vacancies and lattice defects emerged in CuMnOx-MR due to the molecular-scale redox reaction between Cu+ and MnO4−. Meanwhile, the mechanochemical redox process was completed by solvent-free and rapid (1 h) ball milling. Moreover, the ball milling could continually break CuMnOx-MR particles to generate a high degree of interstitial porosity (up to 59 m2 g−1). Compared with the control samples, CuMnOx-MR exhibited a lower catalytic temperature (T90 = 140 °C) in the CO oxidation reaction and performed well in the stability test (150 °C, 100 h). Finally, the CuMnOx-MR showed good resistance to H2O (4.2 vol% moisture, 100 h) and SO2 (100 ppm), which suggests its potential for practical applications.


 Please wait while we load your content...
Please wait while we load your content...