Fundamental interplay between phase-transition kinetics and thermodynamics of manganese-based sodium layered oxides during cationic and anionic redox†
Abstract
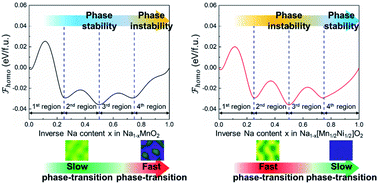
Herein, we present an in-depth understanding of the thermodynamic phase-stability coupled with phase-separation kinetics to rationally utilize the cumulative redox reaction and achieve high-energy density with stable cyclability in Mn-based layered oxides for advanced sodium-ion batteries (SIBs). Based on the mixing enthalpy, Na1−x[Mn1/2Ni1/2]O2 shows faster phase-transition than Na1−xMnO2 in 0.25 ≤ x ≤ 0.75. In x ≥ 0.75, however, Na1−x[Mn1/2Ni1/2]O2 does not evolve the Na-non phase (x = 1.0) transition during charging because of the thermodynamically stable highly Na-poor (HN-poor) phase at x = 0.875. Additionally, the HN-poor phase in Na[Mn1/2Ni1/2]O2 causes less polarization during the anionic redox reaction as compared to the significant voltage-hysteresis at the first charging–discharging in the typical anion-utilized layered cathodes. Our understanding suggests two strategies: (i) increasing vacancy solubility into the Mn–Ni binary oxide during the cation-based redox reaction and (ii) rationally utilizing the HN-poor phase during the anion-based redox reaction, providing new perspectives to achieve the high-energy density with cyclic stability of cathode materials for SIBs.


 Please wait while we load your content...
Please wait while we load your content...