Origin of the enhanced oxygen evolution reaction activity and stability of a nitrogen and cerium co-doped CoS2 electrocatalyst†
Abstract
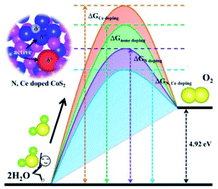
Electrochemical water splitting is a promising technology to enable a sustainable energy supply; however, it remains a formidable challenge to find efficient and stable electrocatalysts. Particularly, oxygen evolution, the half-reaction at the anode, imposes serious overpotential penalties due to its sluggish kinetics. The continued rational design and optimization of OER catalysts via different strategies such as chemical substitution provide an understanding of the way that catalytic properties can be finely tuned. Here, we show a facile synthetic approach involving a Ce(NO3)3 precursor to yield a nitrogen and cerium co-doped CoS2 electrocatalyst. Remarkably, it requires an overpotential of only 190 mV to reach a current density of 10 mA cm−2 and exhibits long-term stability for 120 h. More importantly, a suite of advanced characterization techniques and theoretical calculations reveal complementary roles of the dual dopants which stabilize the intermediates as a result of the formation of two higher oxidation state metal active centers and maintain the surface structure. This work correlates the electronic structure and heteroatom doping, providing insights into electrochemical activity and stability and guiding the rational design of efficient electrocatalysts.
- This article is part of the themed collection: Journal of Materials Chemistry A HOT Papers


 Please wait while we load your content...
Please wait while we load your content...