Optimizing electron density of nickel sulfide electrocatalysts through sulfur vacancy engineering for alkaline hydrogen evolution†
Abstract
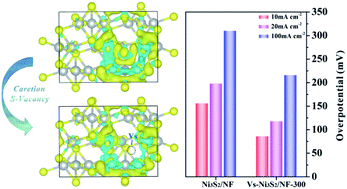
The development of earth-abundant Ni-compound-based electrocatalysts with high performance toward alkaline hydrogen evolution reaction (HER) is of significance for hydrogen generation. Ni3S2 catalysts have been widely regarded as promising candidates, but suffer from unfavorable hydrogen adsorption characteristics; hydrogen absorption on the Ni sites is not sufficiently strong. Herein, by using ab initio calculations, we have shown that hydrogen adsorption on Ni sites of Ni3S2 catalysts can be tuned by introducing sulfur vacancies. Inspired by this theoretical finding, we have fabricated a porous Ni3S2 nanosheet array catalyst grown on nickel foam that is rich in sulfur vacancies (Vs-Ni3S2/NF) by a plasma-assisted method. The catalyst exhibits a much improved HER activity, which only requires a low overpotential of 88 mV at a current density of 10 mA cm−2 and a Tafel slope of 87 mV dec−1 in 1 M KOH. This catalyst also shows high durability, which can sustain 15 h of high-current-density (∼100 mA cm−2) operation with negligible degradation. The theoretical and experimental results reveal that the increase in the electron density of Ni sites induced by sulfur vacancies plays a key role in improving its intrinsic activity. This work opens a new direction for the rational design of highly efficient transition-metal-compound electrocatalysts toward HER and beyond through defect engineering.


 Please wait while we load your content...
Please wait while we load your content...
