Accurate synergy effect of Ni–Sn dual active sites enhances electrocatalytic oxidation of urea for hydrogen evolution in alkaline medium†
Abstract
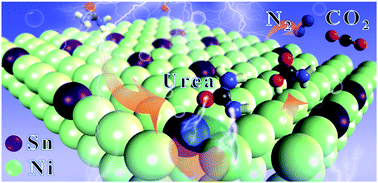
On account of its relatively low equilibrium potential, the electrocatalytic urea oxidation reaction (UOR) has promising application prospects for replacing the oxygen evolution reaction (OER) in boosting the cathode hydrogen production. To overcome the limitation of catalysts with only one active site, Ni–Sn dual sites, respectively pointing at the amino and carbonyl groups of the urea molecule, were designed based on the orbital symmetry matching principle to electrocatalytically oxidize urea at the anode for highly efficient hydrogen evolution at the cathode. The synthesized Ni–Sn sulfide catalyst, having a high active surface area (145 m2 g−1), displayed outstanding performance in the UOR with a low potential of 1.36 V (vs. RHE) at 10 mA cm−2 and achieved a high hydrogen production rate (100 L min−1 gcat−1) at the potential of 1.69 V (vs. RHE). The density functional theory studies combined with Raman experiments unveiled that the enhanced performance is due to the weakening of the urea C–N bond, resulting from the precisely designed Ni–Sn dual active sites, and the self-driven electron transfer between Ni and Sn. The synergistic strategy of point-to-point dual active sites developed here not only achieved a highly efficient UOR to boost hydrogen production, but also provides a new concept when designing catalysts for other catalytic reactions.


 Please wait while we load your content...
Please wait while we load your content...