Role of phosphate source in improving the proton conductivity of tin pyrophosphate and its composite electrolytes†
Abstract
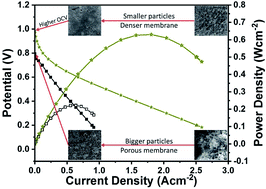
Metal pyrophosphates (MPPs) in general and tin pyrophosphate (TPP) in particular have received significant interest in the last decade due to their potential as proton conductors for electrolyte application in intermediate temperature (IT)-fuel cells. However, for MPP based electrolytes, despite high reported proton conductivities, achieving good fuel cell performance and high open circuit voltage (OCV) remains a challenge with synthesis methods playing a crucial role in determining the final proton conductivity. Here we report the role of phosphate precursor in determining the TPP proton conductivity by examining five different precursors: (1) phosphoric acid (TPP-PA), (2) ammonium hydroxide + phosphoric acid (TPP-NH4OH), (3) diammonium phosphate (TPP-DAP), (4) tetramethylammonium sulphate + phosphoric acid (TPP-TMAP), and (5) tetrabutylammonium phosphate (TPP-TBAP), where a maximum conductivity of 88 mS cm−1 at 200 °C was obtained for TPP prepared from the TBAP precursor. TPP prepared from all of the different precursors formed the crystalline cubic Pa![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) phase after sintering at 650 °C for 2.5 hours. Furthermore, TPP-TBAP/Nafion® composite membranes prepared with a 90 : 10 ratio exhibited an OCV of 0.98 V and produced a maximum peak power density (PPD) of 630 mW cm−2 at an operating temperature of 220 °C. Our results demonstrate the significant impact of the TPP precursor on proton conductivity and fuel cell performance.
phase after sintering at 650 °C for 2.5 hours. Furthermore, TPP-TBAP/Nafion® composite membranes prepared with a 90 : 10 ratio exhibited an OCV of 0.98 V and produced a maximum peak power density (PPD) of 630 mW cm−2 at an operating temperature of 220 °C. Our results demonstrate the significant impact of the TPP precursor on proton conductivity and fuel cell performance.


 Please wait while we load your content...
Please wait while we load your content...
