Enriched pseudocapacitive lithium storage in electrochemically activated carbonaceous vanadium(iv, v) oxide hydrate†
Abstract
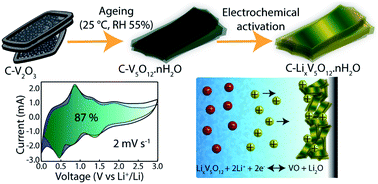
Hydrated vanadium oxides (HVOs) are promising (extrinsic) pseudocapacitive materials that extend the possibility to optimize electrochemical performance through fine-tuning their nanoscale porosity, structural disorder and conventional composition. These materials demonstrate the features of batteries (i.e. high energy density) and supercapacitors (fast power output). However, most pseudocapacitive oxides are characterized by poor conductivity, which leads to high electrode resistance. Herein, an amorphous-like carbon-integrated vanadium oxide hydrate (V5O12·0.4H2O, CHVO) is engineered using a straightforward solvothermal approach, with improved conductivity, rich pore architecture and readily available nanoscale redox-active sites. The CHVO material shows superior performance as an anode in Li-ion batteries with a specific capacity of 1175 mA h g−1 at 50 mA g−1 in the 150th cycle, which was maintained to 525 mA h g−1 over 600 cycles at 1000 mA g−1. The half-cell can be charged to 403 mA h g−1 (1451 C g−1) at 4000 mA g−1 in ∼6 min. The cyclic voltammetry analysis shows that CHVO undergoes an electrochemical activation by forming a stable lithium vanadium oxide in the initial cycles. The research on electrochemical de(lithiation) mechanism of HVOs at low potentials (<1 V) is largely unexplored. In this context, real time measurements using in situ Transmission Electron Microscopy are provided to underpin the Li-ion transport process in CHVO electrodes. It is revealed that the carbon framework and peculiar structure of CHVO effectively buffer large volume expansions upon (de)lithiation. In addition, ex situ X-ray photoelectron spectroscopy and X-ray Diffraction measurements were collectively utilized to uncover a conversion-type reaction mechanism.


 Please wait while we load your content...
Please wait while we load your content...