Solvothermal synthesis of Sn3N4 as a high capacity sodium-ion anode: theoretical and experimental study of its storage mechanism†
Abstract
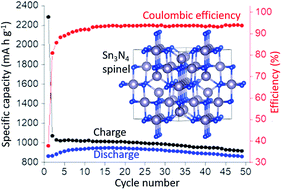
A new simple and scalable method to synthesise spinel-structured Sn3N4 has been developed using SnCl4 and LiNH2 precursors under solvothermal conditions. Nanocrystalline Sn3N4 with a crystallite size < 10 nm was produced and tested as anode material in sodium half cells, demonstrating a very high reversible (de-sodiation) capacity of ∼850 mA h g−1 measured over 50 cycles, the highest reported reversible capacity for a sodium anode apart from sodium itself. Ex situ X-ray absorption spectroscopy and X-ray diffraction show that the electrochemical reactions are reversible and that Sn3N4 is recovered upon re-oxidation. X-ray diffraction shows that the peaks associated with Sn3N4 reflections become narrower during discharge (reduction), evidencing that the smaller Sn3N4 particles are primarily involved in the electrochemical reactions, and broadening of the peaks is reversibly recovered upon oxidation. The analysis of the near edge X-ray absorption data (XANES) shows that the Sn oxidation state decreases during reduction and nearly recovers the initial value during oxidation. DFT calculations suggest that the insertion of Na into the Sn3N4 surface followed by substitution of tetrahedral Sn by Na is energetically favourable, and evidence of the removal of tetrahedral Sn from the spinel Sn3N4 structure is obtained from the analysis of the extended X-ray absorption fine-structure (EXAFS) measurements of reduced electrodes, which also show the recovery of the pristine structure at the end of oxidation. DFT also shows that Sn substitution by Na is only favourable at the Sn3N4 surface (not for bulk Sn3N4), in agreement with the electrochemical characterisation that shows that controlling the nanoparticle size is crucial to achieve full utilisation of Sn3N4 (and thus high capacity).


 Please wait while we load your content...
Please wait while we load your content...