Polymeric carbon nitride with frustrated Lewis pair sites for enhanced photofixation of nitrogen†
Abstract
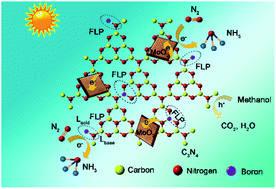
The bottleneck of photocatalytic nitrogen fixation lies in the rate-limiting reductive activation of robust N![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N triple bonds with high bond energy. Herein, polymeric carbon nitride with frustrated-Lewis-pairs was constructed by intercalation of electron-deficient boron (B) into metal-free carbon nitride. NH3-temperature programmed desorption (TPD) and pyridine-adsorption diffuse reflectance infrared Fourier transform spectroscopy (DRIFTS) results demonstrated that B heteroatoms (Lewis acid) and proximal amino groups (Lewis base) were more likely to form frustrated Lewis pairs, which would capture, activate and reduce N2 to NH3 through a “pull–push” effect. Molybdenum-based oxide was further employed as a co-catalyst to improve the charge separation efficiency of site-engineered photocatalysts. The synergistic effect between intercalated B and defective MoO2 resulted in an 8-fold increased photoactivity of carbon nitride for N2 fixation. This work provides a new avenue for the rational design and engineering of frustrated Lewis pair sites on polymeric photocatalysts toward efficient N2 fixation.
N triple bonds with high bond energy. Herein, polymeric carbon nitride with frustrated-Lewis-pairs was constructed by intercalation of electron-deficient boron (B) into metal-free carbon nitride. NH3-temperature programmed desorption (TPD) and pyridine-adsorption diffuse reflectance infrared Fourier transform spectroscopy (DRIFTS) results demonstrated that B heteroatoms (Lewis acid) and proximal amino groups (Lewis base) were more likely to form frustrated Lewis pairs, which would capture, activate and reduce N2 to NH3 through a “pull–push” effect. Molybdenum-based oxide was further employed as a co-catalyst to improve the charge separation efficiency of site-engineered photocatalysts. The synergistic effect between intercalated B and defective MoO2 resulted in an 8-fold increased photoactivity of carbon nitride for N2 fixation. This work provides a new avenue for the rational design and engineering of frustrated Lewis pair sites on polymeric photocatalysts toward efficient N2 fixation.


 Please wait while we load your content...
Please wait while we load your content...