Mixed proton–electron–oxide ion triple conducting manganite as an efficient cobalt-free cathode for protonic ceramic fuel cells†
Abstract
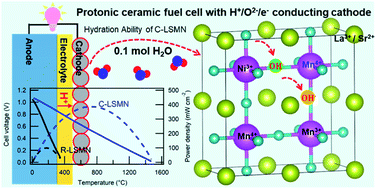
It is challenging for materials chemists to develop efficient, cobalt-free cathode materials for solid oxide fuel cells mainly because of the resource scarcity. This study demonstrates that a cubic-type La0.7Sr0.3Mn0.7Ni0.3O3−δ (C-LSMN7373) perovskite is promising for intermediate-temperature protonic ceramic fuel cells (PCFCs) because of the sufficient H+/e−/O2− triple conductivity. The oxide can be hydrated by gaining 0.1 molar fraction of H2O under wet air at 415 °C, as confirmed by thermogravimetry analysis. An in situ extended X-ray absorption fine structure (EXAFS) analysis shows that the hydration reaction takes place via the association between H2O and oxygen vacancies, coupled with the redox of Mn and O atoms. Rhombohedral-type La0.7Sr0.3Mn1−xNixO3−δ cannot undergo hydration because the oxygen vacancy concentration required for water association is lower than the cubic phase concentration. The cathode performances of various PCFCs are examined by fabricating thin-film cells based on a Ba(Zr0.4Ce0.4Y0.2)O3 electrolyte. The peak power density of the PCFCs with the cubic-type LSMN7373 cathode is 386 mW cm−2 at 600 °C, which is much higher than the reported values for Zr-rich side electrolytes. Moreover, the cathodic polarization resistance is lower than that of a cell with the widely used La0.6Sr0.4Co0.2Fe0.8O3 cathode below 550 °C.


 Please wait while we load your content...
Please wait while we load your content...
