Li-triggered superior catalytic activity of V in Li3VO4: enabling fast and full hydrogenation of Mg at lower temperatures†
Abstract
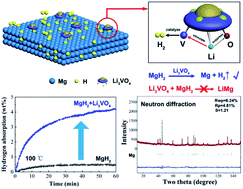
Magnesium-based hydrides are promising hydrogen storage materials; however, little progress has been achieved in improving hydrogen storage performance under ambient conditions, consequently impeding further commercial application. Herein, a lithium rich transition metal oxide, Li3VO4 has been employed to catalyze the hydrogen storage reaction of magnesium-based hydrides, enabling a fast and full hydrogenation of Mg at lower temperatures. The structural stability of Li3VO4 upon de/rehydrogenation reactions has been confirmed by neutron diffraction and transmission electron microscope observations. The synergistic catalytic role of Li3VO4 has elucidated that the existence of Li stimulates the catalytic activity of V and simultaneously suppresses the activity of O. V is the key catalytically active site, while O can react with active Mg to generate MgO which is a passivation layer and could prevent the diffusion of H. These findings will give an opportunity for the use of other lithium rich transition metal oxides in the field of catalyzing hydrogen storage systems.
- This article is part of the themed collection: Journal of Materials Chemistry A HOT Papers


 Please wait while we load your content...
Please wait while we load your content...