Electrochemical CO2-to-CO conversion: electrocatalysts, electrolytes, and electrolyzers
Abstract
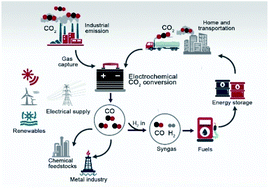
Electrochemical reduction of carbon dioxide (CO2) to value-added chemicals and fuels offers a potential platform to store renewable energy in chemical bonds and thus a route to carbon recycling. Due to its high efficiency and reasonable economic feasibility, the conversion of CO2 to carbon monoxide (CO) is considered as the most promising candidate reaction in the industrial market. Recently, the understanding of the basic mechanism of CO2 reduction to CO has become clearer, which has also motivated the design principles for better-performing catalysts including morphology, size, grain boundary, and surface engineering. Various catalysts (noble and non-noble metals, transition metal chalcogenides, carbon materials, and molecular catalysts) have been developed to efficiently catalyze the CO2-to-CO conversion. Here we survey recent key progress in CO2-to-CO conversion in the field of electrocatalytic CO2 reduction. We will highlight the principles of designing electrocatalysts for the selective formation of CO, the influence of electrolytes on the selectivity and conversion rate, and the emerging applications of electrolyzers for large-scale CO production. We finally provide an outlook on several development opportunities that could lead to new advancements in this promising research field.
- This article is part of the themed collections: Journal of Materials Chemistry A Recent Review Articles and Journal of Materials Chemistry A Emerging Investigators


 Please wait while we load your content...
Please wait while we load your content...