Addressing the OER/HER imbalance by a redox transition-induced two-way electron injection in a bifunctional n–p–n electrode for excellent water splitting†
Abstract
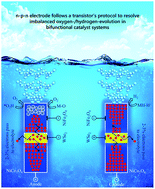
Here, an n–p–n electrode is proposed to preserve the oxygen evolution reaction (OER)/hydrogen evolution reaction (HER) balance without compromising the overall water splitting performance. Electrode activation is synchronized with a photosensitive p-region (WSe2) that forward biases itself with respect to the electrolyte reduction potential. Following activation, electron injection by NiFe2O4 nanodots forms the basis of excellent OER and HER at the catalyst–electrolyte interface. The electron injection potential of NiFe2O4 was confirmed from its ability to function like an organic sensitizer in a solar cell. The photoresponse time and photosensitivity characteristics showed that the catalyst has an n–p–n structure similar to that of a virtual phototransistor. The synergy between electron injection and two-way electron transfer yielded overpotentials of 43 and 92 mV for the HER and OER in alkaline medium, respectively. First principle calculations supported this excellent performance by correlating it to adsorption/desorption binding energy. The results from this research offer a new strategy to design noble metal-free catalyst systems for large-scale solar to fuel generation applications.


 Please wait while we load your content...
Please wait while we load your content...