An in-depth study on the thermodynamics and kinetics of disproportionation behavior in ZrCo–H systems†
Abstract
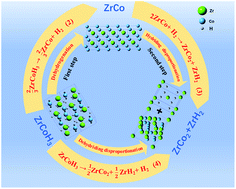
Hydrogen-introduced disproportionation reactions are the main reason for capacity fading in ZrCo–H systems during cycling, but their essential performances, including thermodynamics and kinetics, have so far been inconclusive. Herein, thermodynamic and kinetic investigations of hydriding disproportionation (2ZrCo + H2 → ZrCo2 + ZrH2) and dehydriding disproportionation (ZrCoH3 → ½ZrCo2 + ½ZrH2 + H2) as well as hydrogenation (⅔ZrCo + H2 → ⅔ZrCoH3) and dehydrogenation (⅔ZrCoH3 → ⅔ZrCo + H2) reactions were systematically carried out. Importantly, the thermodynamic equilibrium of hydriding disproportionation was resoundingly sought through a series of discontinuous reactions. Therefore, the hydrogen plateau pressure (0.220 bar at 600 °C) as well as the corresponding enthalpy change ΔH (−123.12 kJ mol−1 H2) and entropy change ΔS (−224.32 J mol−1 K−1 H2) were accurately measured. Moreover, by establishing thermodynamic relationships between the dehydrogenation and hydriding disproportionation reactions, the thermodynamic parameters of dehydriding disproportionation (ΔH = 69.36 kJ mol−1 H2, ΔS = 222.75 J mol−1 K−1 H2) were dexterously determined. To our surprise, it is easier to satisfy the thermodynamic conditions of de-/hydriding disproportionation compared with de-/hydrogenation reactions. Further kinetic studies revealed that de-/hydriding disproportionation reactions require high temperatures (>380 °C) to trigger, because the activation energy is much higher than that of the de-/hydrogenation reactions. Thus, we consider that both disproportionation reactions are inevitable during cycling in terms of thermodynamics. However, the high energy barrier restricts the disproportionation reaction from terminating completely in one cycle, but controls it occurring gradually in each cycle. This research sufficiently explains the attenuation model of cycling capacity in ZrCo–H systems. We suggest that future works focus on increasing the stability of ZrCo–H systems by changing the de-/hydrogenation paths to avoid thermodynamically disproportionation reaction regions.


 Please wait while we load your content...
Please wait while we load your content...