A CO2/H2 fuel cell: reducing CO2 while generating electricity†
Abstract
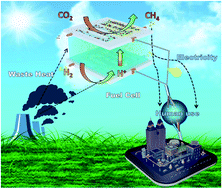
Electrocatalytic conversion of CO2 into hydrocarbons is one of the most promising approaches to reduce the concentration of CO2 in the atmosphere as well as to produce various valuable chemical products from this most prevalent greenhouse gas. However, this process is still many steps away from finding practical application because of its high energy demand. Herein we report an innovative CO2/H2 fuel cell that can convert CO2 into a synthetic fuel (CH4) while generating electricity instead of consuming it. This is unlike conventional electrochemical CO2 reduction cells, which typically consume electricity to reduce CO2. In the new cell, H2 is oxidized to H+ ions (and electrons) at the anode, and the H+ ions then pass through a proton exchange membrane to reduce CO2 to CH4 at the cathode. While doing so, the cell can generate a current density of 94.1 A m−2, a peak power density of 3.9 W m−2 and CH4 at a rate of 75.29 μmol gcat−1 h−1 at 170 °C, a temperature that can be derived from waste heat from various processes. Density functional theory is applied to determine the reaction mechanism on the catalyst in the cell.
- This article is part of the themed collection: Journal of Materials Chemistry A HOT Papers


 Please wait while we load your content...
Please wait while we load your content...