Guided-formation of a favorable interface for stabilizing Na metal solid-state batteries†
Abstract
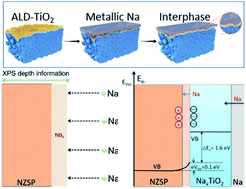
The sodium (Na) anode suffers severe interfacial resistance and dendrite issues in a classic NASICON-type Na3Zr2Si2PO12 (NZSP) electrolyte, resulting in poor electrochemical performance for solid-state Na metal batteries. There has been little success in the reduction of interfacial resistance in recent years. The exact mechanism of this resistance has not been fully understood because of little information about the interface. In this work, we effectively address the large interfacial resistance issue and the metal dendrite problem between the Na anode and NZSP by introducing a TiO2 film as an active interphase. We employ quasiinsitu X-ray photoelectron spectroscopy (XPS) to uncover the interphase formation mechanism at the Na/TiO2–NZSP electrolyte interface. Our quasiinsitu XPS results confirm the formation of a sodiated-TiO2 interphase upon stepwise Na evaporation on the surface of the NZSP electrolyte. Further investigation by molten Na contact angle measurements, impedance spectroscopy and DFT calculations demonstrates that the sodiated-TiO2 interphase promotes Na ion transport between the Na anode and NZSP electrolyte. Moreover, the electrostatic potential formed at the NZSP/NaxTiO2 interface can effectively reduce electronic conductivity at the interface and hence prevent the growth of sodium dendrites. A representative paradigm for interphase design is provided to address the interface contact for developing stable solid-state batteries with high performance.


 Please wait while we load your content...
Please wait while we load your content...