Hierarchically porous nickel–iridium–ruthenium–aluminum alloys with tunable compositions and electrocatalytic activities towards the oxygen/hydrogen evolution reaction in acid electrolyte†
Abstract
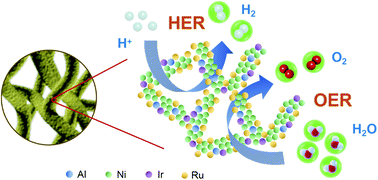
Developing efficient and less noble-metal-dependent electrocatalysts toward both the oxygen evolution reaction (OER) and hydrogen evolution reaction (HER) is one of the key challenges for polymer electrolyte membrane (PEM) electrolyzer applications. Herein, we report the preparation of bifunctional NiIrRuAl multimetallic nanoporous nanowires (NPNWs) with different compositions through the combination of metallurgical control and a two-step dealloying method. The synthesized NiIrRuAl NPNWs display ultrahigh OER and HER activities in acid solution. With a significantly reduced noble metal (Ir and Ru) content as low as 34 at%, the NiIrRuAl-1/3 electrocatalyst (Ir : Ru = 1 : 3) yields the best OER performance, showing an extremely low overpotential of 237 mV at 10 mA cm−2, and the overpotential of the NiIrRuAl-3/1 electrocatalyst (Ir : Ru = 3 : 1) in the HER is only 14 mV at 10 mA cm−2 in 0.1 M HClO4 solution. More significantly, the NiIrRuAl NPNWs only need a cell voltage of 1.464 V to reach 10 mA cm−2 for overall water splitting and show excellent long-term stability.


 Please wait while we load your content...
Please wait while we load your content...