Polysulfide entrapment and retardation in gel electrolyte Li–S batteries: experiments and modeling†
Abstract
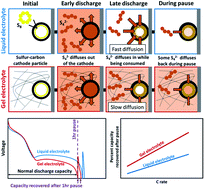
Gelling the electrolyte in lithium–sulfur (Li–S) batteries provides advantages in terms of safety and mitigating polysulfide shuttling leading to reduced side reactions and dendrite growth on the anode side. In the current study, organic and ceramic crosslinkers have been used to improve the entrapment of polysulfides and prevent them from shuttling to the anode side. However, the performance of gel electrolytes is still far from being on par with liquid electrolytes in terms of raw capacity. The discharge curves exhibit an extended first plateau for gel electrolyte batteries compared to liquid electrolytes, showing that polysulfides are better trapped in the gel system. Yet, in the conversion of soluble polysulfides into insoluble polysulfides, the second plateau was delayed. Qualitative and quantitative analysis of the polysulfide diffusion in the liquid electrolyte and gel electrolyte systems were also conducted to predict the relative diffusivity ratio between the liquid electrolyte and gel electrolyte for simulation. To probe what happens at the end of the discharge, where the mass transport in the gel electrolyte appears to be slow, a modified discharge procedure was used where the cell is first paused after discharge and then discharged a second time before recharging normally. Coin cell tests for two gel electrolytes in addition to liquid electrolyte show up to 7% capacity recovery after the pause, which indicates that the diffusion of species during the pause is responsible for the failure recovery. Additionally, the recovery is higher for gel electrolytes compared to liquid, for higher C-rates, and for longer pause times. To better understand this behavior, a Li–S numerical model with mass transport limited reactions was used to examine the polysulfide diffusion coefficients expected in different electrolyte systems. The model is able to reproduce the trends seen in experiments and yields a higher recovery for smaller Li2S4 diffusion coefficients, suggesting that insufficient Li2S4 mass transport is responsible for failure at the end of discharge. By identifying the effect that gel electrolytes have on the state of the battery during discharge using both experimental and simulation methods, this work is a step towards leveraging the advantages of gel electrolytes and mitigating their weaknesses.


 Please wait while we load your content...
Please wait while we load your content...
