Engineering metal sulfides with hierarchical interfaces for advanced sodium-ion storage systems†
Abstract
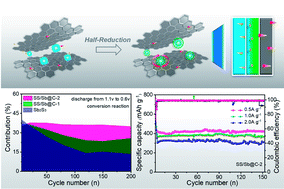
Antimony sulfide as an energy storage material with remarkable theoretical capacity has captured the attention of several researchers, but it has disadvantages such as volume expansion, polysulfide dissolution, and sluggish kinetics. By utilizing the oxygen functional groups in phenolic resin, engineering the nature mineral Sb2S3 with hierarchical interfaces (Sb, S-doped carbon) can be undertaken, effectively facilitating the diffusion of ions and accommodating volume changes. Importantly, the double-controlling synergistic effects of the Sb shell and S-doped carbon would prolong the diffusion pathway of polysulfides, considerably enhancing the sodium-ion storage capability. As a result, a capacity retention rate of 97.1% at 0.1 A g−1 could be obtained after 200 cycles. At 0.5, 1.0, and 2.0 A g−1 after 150 cycles, capacities of 422.6, 367, and 311.1 mA h g−1, respectively, could be retained. A detailed investigation regarding capacity confirmed that the introduced hierarchical interfaces could significantly promote the faster transfer of electrons and trapping of polysulfides accompanied with improved reversible conversion reactions. The quantitative analyses of capacitive contribution and EIS data revealed that the core–shell structure and S-doped carbon could fundamentally boost the pseudocapacitive behaviors with improved transfer of electrons/ions. This rational design is expected to brighten the prospects for designing metal sulfides for use as advanced anodes in sodium-ion batteries.


 Please wait while we load your content...
Please wait while we load your content...