A chlorinated nonacyclic carbazole-based acceptor affords over 15% efficiency in organic solar cells†
Abstract
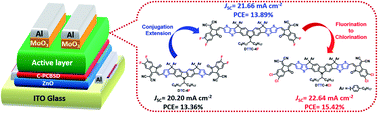
In this contribution, a dithienocyclopentacarbazole (DTC)-based and two dithieno[3,2-b]thiophenecyclopentacarbazole (DTTC)-based non-fullerene acceptors (NFAs) named DTC-4F, DTTC-4F and DTTC-4Cl were exploited to elucidate the effects of conjugation extension and end group chlorination. DTTC-4F was designed through conjugation extension on the basis of DTC-4F by fusing one additional thiophene on both flanks of the heptacyclic DTC core, generating the nonacyclic DTTC core. Compared with DTC-4F, DTTC-4F features up-shifted energy levels, red-shifted absorption and enhanced π–π interaction. PM6:DTTC-4F exhibits a decent PCE of 13.89% with a VOC of 0.95 V, a JSC of 21.66 mA cm−2 and a FF of 67.60%. Although DTTC-4F affords a reduced FF compared to DTC-4F, a DTTC-4F-based device delivers a higher PCE than DTC-4F-based devices due to the extended absorption range of DTCC-4F in comparison with DTC-4F. Since chlorinated NFAs are known to possess stronger π–π interaction than fluorinated NFAs, DTTC-4Cl was therefore synthesized by end-capping DTTC core with 2Cl-IC groups instead of 2F-IC groups. Moreover, DTTC-4Cl demonstrates a red-shifted absorption in comparison with DTTC-4F, which is beneficial for light-harvesting. Overall, PM6:DTTC-4Cl affords an outstanding PCE of 15.42% with a VOC of 0.92 V, a JSC of 22.64 mA cm−2 and a FF of 74.04%, which is the record PCE observed in carbazole-based NFAs.
- This article is part of the themed collections: 2020 Journal of Materials Chemistry A most popular articles and Journal of Materials Chemistry A Lunar New Year collection 2021


 Please wait while we load your content...
Please wait while we load your content...