A dual-salt coupled fluoroethylene carbonate succinonitrile-based electrolyte enables Li-metal batteries†
Abstract
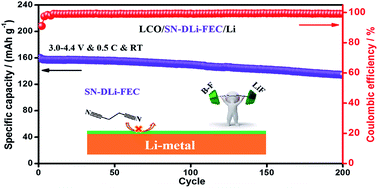
Succinonitrile (SN)-based electrolytes are attractive due to their considerable ionic conductivity and high oxidative stability. However, the spontaneous chemical side-reactions between SN and Li-metal restrict the extensive applications of such electrolytes in next-generation advanced Li-metal batteries. Herein, we report a novel SN-based electrolyte SN–DLi–FEC which combines the dual-salt (lithium bis(trifluoromethane sulfonimide) (LiTFSI) and lithium difluoro(oxalato)borate (LiODFB)) and the fluoroethylene carbonate (FEC) additive. Compared with other SN-based electrolytes such as SN–SLi–FEC (single salt combined with FEC), SN–DLi–FEC can form a more stable and durable organic–inorganic composite SEI film rich in the B–F bond and LiF on the surface of the Li-metal anode. As a result, interfacial compatibility of the SN-based electrolytes and the Li-metal anode can be significantly enhanced. Moreover, SN–DLi–FEC shows high oxidation resistance, high ionic conductivity and good thermal stability. The LiCoO2 (LCO)/Li battery equipped with SN–DLi–FEC exhibits excellent electrochemical performances at room temperature (RT) and high temperature, and high thermal safety. The novel SN-based electrolyte prepared in this work could be a promising candidate providing better reliability and safety for the development of Li-metal batteries.
- This article is part of the themed collection: Battery science and technology – powered by chemistry


 Please wait while we load your content...
Please wait while we load your content...