LiSi3As6 and Li2SiAs2 with flexible SiAs2 polyanions: synthesis, structure, bonding, and ionic conductivity†
Abstract
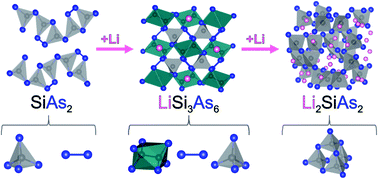
Two novel ternary phases, LiSi3As6 and Li2SiAs2, have been synthesized and characterized. Both phases have an identical Si : As ratio of 1 : 2 providing insight on how layers of the parent phase SiAs2 accommodate excess electrons from Li cations to form Si–As anionic frameworks. LiSi3As6 exhibits a variety of bonding schemes involving Si–Si and As–As bonds, as well as corner-sharing SiAs4 tetrahedra, while Li2SiAs2 is isostructural to the previously reported Li2SiP2, with adamantane-like Si4As10 units connected into 3D framework. LiSi3As6 and Li2SiAs2 are predicted to be indirect semiconductors which was experimentally confirmed by optical properties characterization. Li2SiAs2 exhibits low thermal conductivity of 1.20 W m−1 K−1 at 300 K in combination with a room temperature ionic conductivity of 7 × 10−6 S cm−1, an order of magnitude greater than that of the phosphide and nitride analogues, indicating its potential as a solid-state Li-ion conductor.


 Please wait while we load your content...
Please wait while we load your content...