A novel single-atom catalyst for CO oxidation in humid environmental conditions: Ni-embedded divacancy graphene†
Abstract
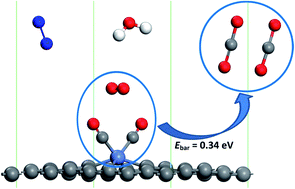
The degradation of catalysts for CO oxidation in humid air is a common issue owing to the blocking of the active site by the adsorption and dissociation of water molecules. In order to evaluate the effect of humidity on the CO oxidation, the adsorption and dissociation of the common gas molecules in air, namely CO, O2, H2O, and N2, on single Ni-embedded divacancy graphene (Ni-DG) have been investigated using first-principles calculations. It was found that all the molecules keep their molecular state and the adsorption energy of CO is much larger than those of the other molecules. In addition, the CO molecules can sufficiently substitute the pre-adsorbed O2, H2O, and N2 molecules with small energy barriers, indicating that the active site of the Ni-DG will not be blocked by water molecules in humid environments. At most two CO molecules can be chemically adsorbed on the Ni-DG, indicating that a new termolecular Eley–Rideal (TER) mechanism is preferred, and the energy barrier for the rate-limiting step (2CO + O2 → OCOOCO) is only 0.34 eV. Hirshfeld charge analysis shows that the charge transfer from the O2-2π* orbital to the CO-2π* orbital plays an important role in the CO oxidation via the TER mechanism. Overall, our results show that the low-cost Ni-DG is an efficient catalyst for CO oxidation, even in humid air at low temperature.


 Please wait while we load your content...
Please wait while we load your content...