Cost-effective porous-organic-polymer-based electrolyte membranes with superprotonic conductivity and low activation energy†
Abstract
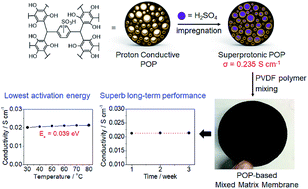
For real-world applications of proton exchange membrane fuel cells (PEMFCs), potential electrolyte materials with high proton conductivity should possess activation energy as low as possible so that good conductivity can be achieved even at low operating temperatures. It is desirable and yet challenging to devise such materials with very low activation energy (<0.1 eV) while maintaining high conductivity. In this work, we have prepared phloroglucinol-based porous organic polymers (POPs) 1S1M, 1S2M, and 1S3Mvia post-synthetic impregnation of sulfuric acid into the pores. The superprotonic conductivity of 1S1M is 2.35 × 10−1 S cm−1 at 70 °C and 90% relative humidity (RH), with a significantly low activation energy of 0.075 eV. The conductivity exceeds that observed for POP-based conductors and is even superior to that of Nafion. Proton-conducting mixed matrix membranes (1S1MP, 1S2MP, and 1S3MP) composed of these powders and poly(vinylidene fluoride) have been fabricated via a drop casting method. 1S3MP exhibits a high proton conductivity of 2.13 × 10−2 S cm−1 at 80 °C and 90% RH. To the best of our knowledge, the activation energy (0.039 eV) of 1S3MP is the lowest recorded value among those for any proton conductive materials. The membrane, which is easy to fabricate and scalable for mass production, is durable over 3 weeks with invariant conductivity.


 Please wait while we load your content...
Please wait while we load your content...