Aerobic selective oxidation of methylaromatics to benzoic acids over Co@N/Co-CNTs with high loading CoN4 species†
Abstract
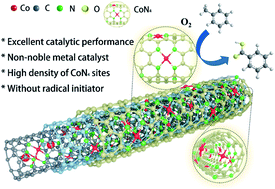
Selective oxidation of toluene to benzoic acid (BAC) with benign oxidant O2 is of great importance, but remains a great challenge, especially using non-noble metallic catalysts. In this work, the Co@N/Co-CNT catalyst, which was composed of atomically dispersed CoN4 species embedded in N-doped CNTs and encapsulated Co nanoparticles, was synthesized. The loading CoN4 could reach 6.4 wt%. It was found that the catalyst exhibited outstanding performance for selective aerobic oxidation of toluene to BAC without additives under solvent-free conditions. The selectivity of BAC could be as high as 91.6% at a toluene conversion of 33.5%, which is much higher than the value reported to date, including those over noble metal-based catalysts. The control experiments indicated that the main active species were CoN4 sites and the outstanding catalytic performance of Co@N/Co-CNTs can be attributed mainly to the unique properties of the CoN4 species and its high loading in the catalyst. Furthermore, the Co@N/Co-CNTs also had an excellent catalytic performance in the selective aerobic oxidation of xylenes to toluic acid derivatives.


 Please wait while we load your content...
Please wait while we load your content...