Probing into the origin of an electronic conductivity surge in a garnet solid-state electrolyte†
Abstract
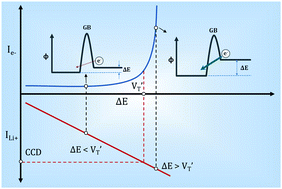
Herein, through studying the electronic conductivity of a garnet electrolyte Li7La2.75Ca0.25Zr1.75Nb0.25O12 (LLCZN) at different temperatures, a model to describe the electron transfer process within garnet electrolytes is proposed for the first time. In this model, electronic conductivity is mainly determined by the barrier height and bias between the grain boundaries of LLCZN. As the external voltage polarization increases with ion current densities and finally exceeds a threshold value, electron conductivity concentration at grain boundaries increases sharply to the critical point for the combination between Li-ions and electrons. Lithium metal will consequently deposit on the grain boundaries and short circuit LLCZN. According to the results, lowering the voltage polarization across the solid electrolyte and increasing the electron transfer energy barrier at grain boundaries are two effective approaches to realize practical applications of garnet electrolytes in solid-state lithium metal batteries.


 Please wait while we load your content...
Please wait while we load your content...