Cr-doped lithium titanate nanocrystals as Mg ion insertion materials for Mg batteries†
Abstract
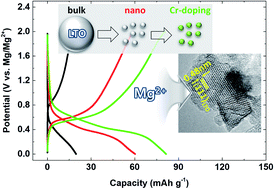
Magnesium metal anodes offer significant economic, capacitive, and safety advantages over their lithium counterparts. However, developing viable magnesium batteries remains challenging because of the lack of efficient insertion cathode materials; the presently used materials suffer from lethargic reaction kinetics caused by strong electrostatic interactions between the divalent intercalant ions and the host. Herein, we present a technique integrating physical and chemical approaches toward achieving high intercalation kinetics utilizing lithium titanate (LTO). We demonstrate that reducing the particle size of LTO to 8 nm drastically enhances the utilization of redox-active centers, leading to a 300% higher reversible capacity than the bulk-sized material. Moreover, aliovalent doping of Cr into the LTO structure not only increases the electrical conductivity, facilitating fast charge redistribution at redox centers, but also causes a favorable structural disordering that can enhance the local ion mobility, ensuring good high-rate performance, long–term cycle stability, and rapid completion of initial activation compared to that offered by the undoped LTO. These findings agree with the computational study reporting that the diffusion barriers for Mg2+ and Li+ ions can be reduced significantly upon Cr doping. This study provides an interesting and novel opportunity to improve conventional insertion materials with poor reaction kinetics to unlock excellent electrochemical activity for practical application in magnesium battery cathodes.


 Please wait while we load your content...
Please wait while we load your content...