Selective acid leaching: a simple way to engineer cobalt oxide nanostructures for the electrochemical oxygen evolution reaction†
Abstract
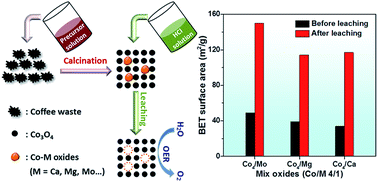
Developing a simple and cost-effective strategy to construct earth-abundant catalysts is in high demand for diverse applications. Herein, a general and facile strategy is developed to engineer cobalt oxide nanostructures via selective acid leaching for the electrochemical oxygen evolution reaction (OER). A leaching process is implemented to selectively remove CoMoO4 by treating mixed Co–Mo oxides in diluted hydrochloric acid solution, resulting in the formation of sub-5 nm particles and a threefold increase in the specific surface area (up to 150 m2 g−1). The leached oxides exhibit superior OER activity to pristine oxides as a result of (i) a larger surface area, (ii) phase purification to expose more active Co3O4 species to the reactant, and (iii) faster charge transfer kinetics for the OER. This strategy can be also applied to a broader range of earth-abundant metals, where a second metal (Li, Ca, and Mg) is selectively leached out, which results in a material with a larger surface area and enhanced catalytic performance for the OER. Moreover, various metal oxides with a high surface area, such as NiO and Fe2O3, can be prepared via this simple synthetic method. This work will pave a new practical way for the production of high surface area catalysts for diverse applications.


 Please wait while we load your content...
Please wait while we load your content...