Electronic reconfiguration of Co2P induced by Cu doping enhancing oxygen reduction reaction activity in zinc–air batteries†
Abstract
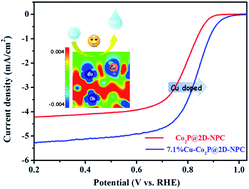
Developing highly efficient and low-cost bifunctional electrocatalysts towards the oxygen reduction reaction (ORR) is important for Zn–air batteries (ZABs) but still a challenge. In this work, electronic reconfiguration and structural optimization are simultaneously conducted to enhance the oxygen reduction reaction (ORR) electrocatalytic activity of Co2P nanoparticles, which were doped with Cu and confined within 2D ultrathin N,P-codoped carbon (NPC) nanosheets. Density functional theory calculations reveal that Cu doping can lead to more positive sites at the neighbouring Co site and weaken the binding force between the surface active sites and the adsorbed intermediates, while the 2D ultrathin nanosheets with Cu doped Co2P nanoparticles tightly anchored can remarkably boost the surface exposure of actives sites endowing such an architecture with efficient mass and charge transport pathways. As expected, the optimized Cu doped Co2P on 2D ultrathin N,P-codoped carbon (Cu-Co2P@2D-NPC) with 7.1% Cu doping exhibits a higher half-wave potential of 0.835 V than Pt/C (0.830 V). Meanwhile, flexible solid state ZABs assembled based on the 7.1%Cu-Co2P@2D-NPC hybrid deliver a high power density of 52.5 mW cm−2 and 32 h stability in the flat and folded states. This promising work holds significant potential to fabricate an efficient electrocatalyst for flexible solid state ZABs and further understand the relationship between the structure and activity.


 Please wait while we load your content...
Please wait while we load your content...