Visualizing spatial potential and charge distribution in Ru/N-doped carbon electrocatalysts for superior hydrogen evolution reaction†
Abstract
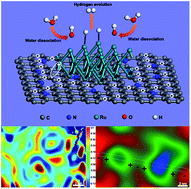
Ruthenium (Ru) has recently emerged as a promising alternative to Pt for the electrocatalytic hydrogen evolution reaction (HER). However, it remains a great challenge to design highly active and durable Ru-based electrocatalysts, and the intrinsic electrocatalytic mechanism is rarely studied in detail. Here a facile one-step pyrolysis strategy is reported to synthesize ultrafine Ru nanocrystals uniformly dispersed on N-doped carbon (Ru/NC) as electrocatalysts for the alkaline HER. The Ru/NC electrocatalysts exhibit a small overpotential of 17 mV at a current density of −10 mA cm−2 and a low Tafel slope of 32 mV dec−1 as well as excellent durability in 1 M KOH, outperforming commercial Pt/C. Density functional theory calculations indicate that a decreased H2O dissociation energy barrier and optimal Gibbs free energy for hydrogen adsorption can be achieved. Importantly, an off-axis electron holography is utilized for the first time to visualize electrostatic potential and charge distribution at the interfaces in Ru/NC, which may promote the charge transfer and facilitate hydrogen adsorption/desorption kinetics, resulting in superior HER performance. This work not only offers a simple and effective method for the synthesis of Ru-based HER electrocatalysts, but also provides indepth insights into the underlying mechanism toward the alkaline HER.


 Please wait while we load your content...
Please wait while we load your content...