Photoinduced formation of Cu@Cu2O@C plasmonic nanostructures with efficient interfacial charge transfer for hydrogen evolution†
Abstract
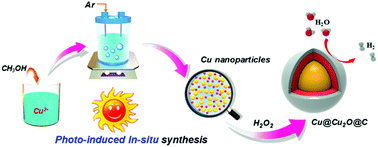
Semiconducting oxide encapsulated plasmonic-metal nanostructures have the advantage of a wide spectral response by combining intrinsic light absorption and resonant energy transfer over their individual components. However, these nanostructures, which are usually prepared by a series of complex steps under harsh conditions, still suffer from inefficient interfacial charge transfer in (photo)-electrochemical and photocatalytic applications because of the degradation and photocorrosion of narrow-band-gap semiconductors. Here, we demonstrate that carbon-encapsulated Cu@Cu2O nanostructures can homogeneously grow through stepwise spontaneous formation from a cupric ion-containing aqueous alcoholic solution induced by irradiation. The unique Cu@Cu2O@C nanostructures in situ synthesized from photocatalytic systems for hydrogen generation can significantly improve solar-light harvesting, effectively prevent Cu2O photocorrosion, and greatly reduce the trap states to reduce charge recombination. By virtue of these merits, the Cu@Cu2O@C nanostructures exhibit efficient and stable hydrogen generation for both (photo)-electrochemical and photocatalytic applications.


 Please wait while we load your content...
Please wait while we load your content...