Layered metal vanadates with different interlayer cations for high-rate Na-ion storage†
Abstract
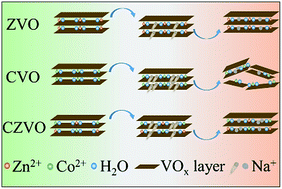
Layer-structured metal vanadates have been regarded as promising candidates for high-rate Na-ion storage. However, without a detailed understanding of the relationship between the interlayer metal ions and the cycling performance, it remains a difficult task to systematically explore layered metal vanadates as high performance electrode materials. Herein, a series of metal vanadates with different interlayer cations such as Co2+ and Zn2+ are prepared and applied as Na-ion battery anodes. First principles simulations and ex situ X-ray diffraction measurements demonstrate that the Na-ion storage performance of the layered metal vanadates is closely related to the structural stress induced by Na+ insertion, and the ion diffusion barrier, as well as the structural reversibility. In addition, a double-interlayer-cation metal vanadate, i.e., Co0.16Zn0.09V2O5·nH2O, is reported for the first time as a high-rate Na-ion battery anode. This compound successfully combines the favorable features of Co0.25V2O5·nH2O and Zn0.25V2O5·nH2O, resulting in the best cycling performance. CV analysis and operando X-ray diffraction measurements reveal a large pseudocapacitive contribution and small volume change of Co0.16Zn0.09V2O5·nH2O during cycling. Our study presents a versatile concept for the optimization of metal vanadates for Na-ion storage, which may open a promising direction for developing high-rate energy storage materials.


 Please wait while we load your content...
Please wait while we load your content...