Cu supported on polymeric carbon nitride for selective CO2 reduction into CH4: a combined kinetics and thermodynamics investigation†
Abstract
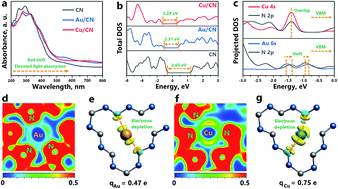
Metal-based cocatalysts have been widely applied in the photocatalytic CO2 reduction reaction (CO2RR) and the integration of cocatalysts/semiconductors is expected to enhance the selectivity of the catalytic reaction. Nevertheless, the type and yield of products manifest a large discrepancy even for the same cocatalyst/semiconductor photocatalytic system, which requires a comprehensive study into the reaction mechanism and product distribution in detail. Here we provide kinetics evidence that selective CO2 reduction into CH4 is realized on Cu loaded carbon nitride (Cu/CN), in comparison with that on Au species (Au/CN) which yield both CO and CH3OH. Besides, it is revealed that the adsorption/desorption thermodynamic behavior of CH3OH is a crucial factor to determine the product distribution. By the establishment of binary electron transfer channels at the interface between adsorbed CH3OH and the Cu/CN surface, intensified activation of CH3OH is accomplished, resulting in the over-all reduction of CO2 into methane. Owing to the combined kinetics and thermodynamics investigation at the atomic level, this work may provide pioneering design schemes of precisely tailored cocatalyst/semiconductor photocatalytic systems for the selective CO2RR.


 Please wait while we load your content...
Please wait while we load your content...