NiFe (sulfur)oxyhydroxide porous nanoclusters/Ni foam composite electrode drives a large-current-density oxygen evolution reaction with an ultra-low overpotential†
Abstract
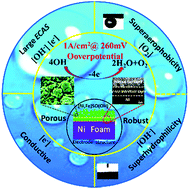
A robust, superwetting and conductive amorphous NiFe (sulfur)oxyhydroxide porous nanoclusters/Ni foam composite electrode was fabricated using a facile one-step wet synthesis method. The S-incorporation strategy enhances the electron transfer and wetting performance of NiFe oxyhydroxide, and dramatically improves the oxygen evolution reaction (OER) performance. This catalytic electrode can drive a 1A cm−2 OER with the lowest reported overpotential of 260 mV and stably drive a 1 A cm−2, and a higher 3 A cm−2 OER process in 1 M KOH. 3 A cm−2 is the highest current density value recorded for a stably driven long-term OER process to the best of our knowledge. Inspired by the intrinsic OER equation, this study highlights that the conductive, large electrochemically active surface area (ECAS) and superwetting properties are important factors for designing large-current-density OER catalysts.


 Please wait while we load your content...
Please wait while we load your content...