Mechanism of ion conductivity through polymer-stabilized CsH2PO4 nanoparticular layers from experiment and theory†
Abstract
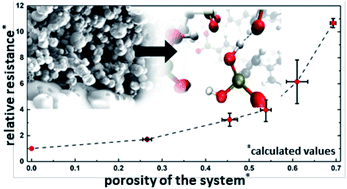
Electrodes are currently the primary performance-limiting component in low and intermediate temperature fuel cells. A proven method for improving electrode performance in solid acid fuel cells is to create ever finer nanostructures and thus increase the electrochemically-active surface area. However, this performance enhancement is limited by issues of long-term stability, as well as increasing both the electronic and ionic conduction pathways. Here, we combine a systematic experimental study with a computational model to quantify the effect of (1) the stabilizing polymer polyvinylpyrrolidone as well as (2) the porosity and electrode layer thickness on the average ionic conductivity of the solid acid electrolyte CsH2PO4 in a composite solid acid fuel cell electrode. With a multiscale simulation approach using a combined molecular dynamics and lattice Monte Carlo method, proton conduction through a porous electrode is simulated at mesoscopic timescales while retaining near-atomistic structured evolution. Electrochemical impedance spectroscopy is used to evaluate the porous electrodes obtained via spray drying. Both approaches reveal a similar and significant contribution of the porous electrolyte layer to the overall cell resistance. This indicates that geometrical parameters, as well as stabilizing materials may play an essential role when designing a high-performance solid acid fuel cell.


 Please wait while we load your content...
Please wait while we load your content...