Passivation of aluminum current collectors in non-aqueous carbonate solutions containing sodium or potassium hexafluorophosphate salts
Abstract
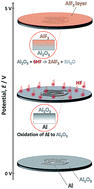
In this study, the investigation of the electrochemical behavior and passivation process of Al metal as a current collector for non-aqueous sodium-ion and potassium-ion batteries is performed. Electrochemical polarization in dynamic mode, in situ scratch polarization in transient mode, and surface analysis using time-of-flight secondary-ion mass spectroscopy (ToF-SIMS) are applied to understand the passivation of aluminum in fluorine-containing non-aqueous alkyl carbonate solutions. Polarization toward the cathodic direction does not result in the formation of alloys between Na or K metals and aluminum even at 0 V vs. Na+/Na or K+/K, respectively. For anodic polarization, the passivation of aluminum in the electrolyte is associated with the occurrence of an anodic reaction. ToF-SIMS surface analysis reveals that the passive layer is composed of an outer AlF3 layer on the Al2O3 layer. The formation of the AlF3 layer occurs via the reaction of Al2O3 with HF, Al2O3 + 6HF → 2AlF3 + 3H2O, as HF is produced as a byproduct of the oxidative decomposition of NaPF6 or KPF6 salts at high potential. The AlF3 passive layer is stable up to 5 V vs. Na+/Na or K+/K, with improved stability observed in the Na solution relative to that in the K electrolyte.


 Please wait while we load your content...
Please wait while we load your content...